What Element Has 26 Protons and 23 Electrons
The element helium for example contains atoms with two protons in the nucleus. The numbers after the decimal point represent the usually very small mass of the.

Solved 1 The Fe3 Ion Has A 26 Electrons 26 Protons And Chegg Com
Compared to atoms of the same element isotopes have different A.

. Example 1 - Numbers of Protons in Gold. Periodic Table of Elements 1. Shapes and Centers of Distributions.
Element Atomic Mass Protons Neutrons Electrons Symbol. Subtract the atomic number from the atomic mass. The neutron is a subatomic particle symbol n or n 0 which has a neutral not positive or negative charge and a mass slightly greater than that of a protonProtons and neutrons constitute the nuclei of atomsSince protons and neutrons behave similarly within the nucleus and each has a mass of approximately one atomic mass unit they are both referred to as nucleons.
Protons and Neutrons in Magnesium. Atoms must also have equal numbers of protons and electrons So if we know the atomic number of an element then we also know how many protons in an element. The element iron contains atoms with 26 protons.
Youll find more specific groups like transition metals rare earths alkali metals alkaline earth halogens and noble gasses. A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus for example carbon-13 with 6 protons and 7 neutrons. Categorical Data on Two Variables.
The atomic number is based on the number of protons in the atom of an element. Describing Variability and Comparing Distributions. When given the protons and electrons indicate the ion with the correct charge.
The element oxygen contains atoms with eight protons. Toggle Module 2 Module 2. Toggle Topic A Topic A.
Types of subatomic particles. Gold silver helium oxygen mercury hydrogen sodium nitrogen niobium neodymium chlorine carbon. It is by mass the most common element on Earth forming much of Earths outer and inner core.
Toggle Topic C Topic C. The first one has been done for you. Periodic Table of Elements 3.
Charged Objects as an Imbalance of Protons and Electrons. A typical atom consists of a nucleus and electron cloudAtom components are positively-charged protons and electrically neutral neutrons in the nucleus and negatively-charged electrons orbiting this nucleus. Since the vast majority of an atoms mass is made up of its protons and neutrons subtracting the number of protons ie.
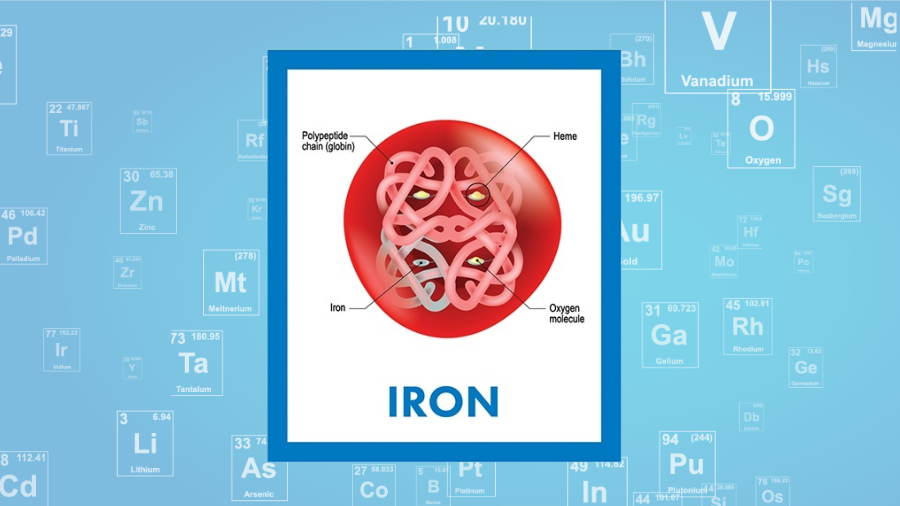
The chemical symbol for Iron is Fe. The element Gold Symbol Au has the Atomic Number of 79. Protons and neutrons are called nucleons.
The nuclide concept referring to individual nuclear species emphasizes nuclear properties over chemical properties whereas the isotope concept grouping all atoms of each element emphasizes. Atom is the smallest constituent unit of matter that retains the properties of an element. Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure.
The atomic number from the atomic mass will give you the calculated number of neutrons in the atom. Ion Protons Electrons Protons Electrons Ion Cl1- 56 54 K1 87 86 S2- 84 86 Sr2 50 46 Al3 32 36 P3- 55 54 Si4- 12 10 Use your periodic table to complete the table below. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol ZThe total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19 coulombs.
Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Toggle Topic B Topic B. It is the fourth most common element in.
In the previous section of Lesson 1 an atom was described as being a small and dense core of positively charged protons and neutral neutrons surrounded by shells of negatively charged electronsThe protons are tightly bound within the nucleus and not removable by ordinary measures. Iron is a metal in the first transition series. There are multiple ways of grouping the elements but they are commonly divided into metals semimetals metalloids and nonmetals.

Solved What Is The Name And Symbol Of An Ion That Has 26 Protons And 23 Electrons Fe 3 Fe 2 Mn 2 Mn 3

Solved This Element Symbol 523 Fe Has 26 O A 23 Electrons Chegg Com
Comments
Post a Comment